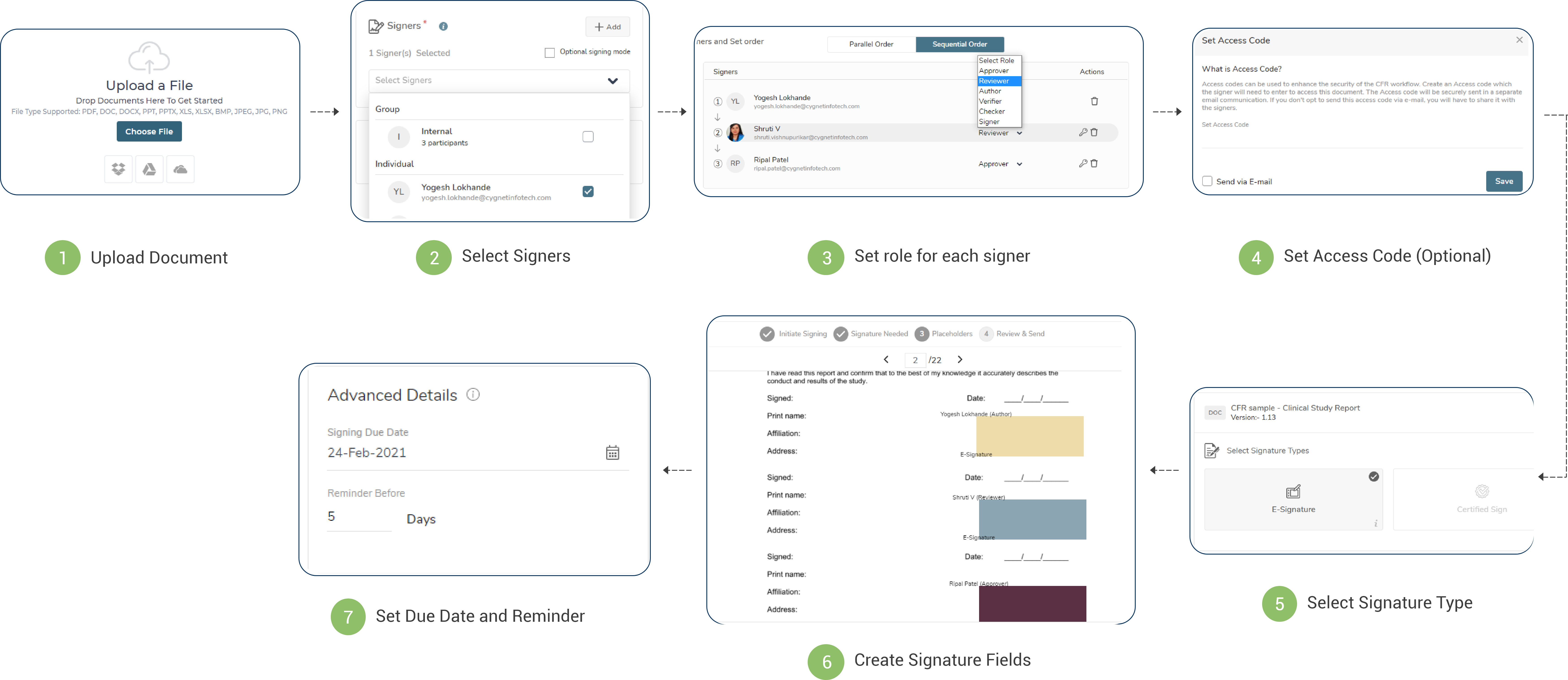
What is 21 CFR - Part 11 compliance?
The title 21 CFR [Code of Federal Regulations] PART 11 is a regulation act established by United States’ FDA [Food & Drug Administration] for electronic records and electronic signatures. This compliance defines the criteria under which the e-Signatures and electronic records are considered equivalent to the paper records and the hand-written wet signatures. This regulation caters to the security concerns about the management, storage and retrieval of the electronic records and signatures by users of industries like biotechnology, pharma, and medical equipment manufacturers in this digital age.

Cygnature and 21 CFR compliance
Cygnature being an electronic and digital signing tool, needs to fulfil the criteria to comply with the 21 FDA CFR regulations and Cygnature secures the workflow creation, signing and user authentication modules. This makes the signing process even more secure with multiple authentication layers and captures audit trials and timestamps of signing.

Features of 21 CFR - Part 11 module
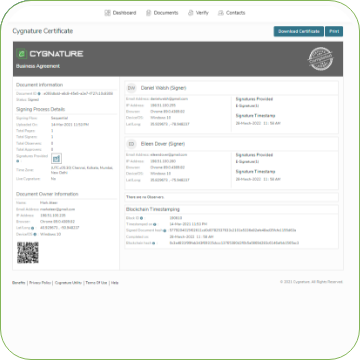
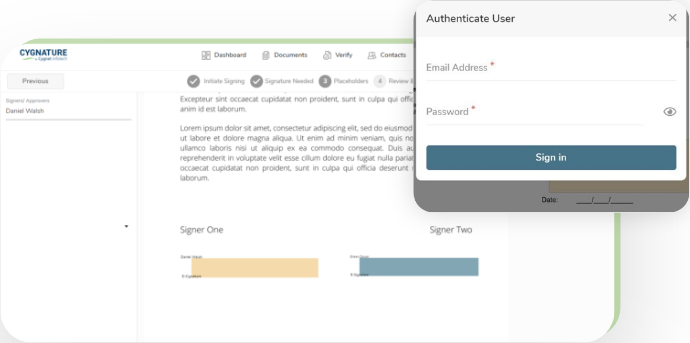
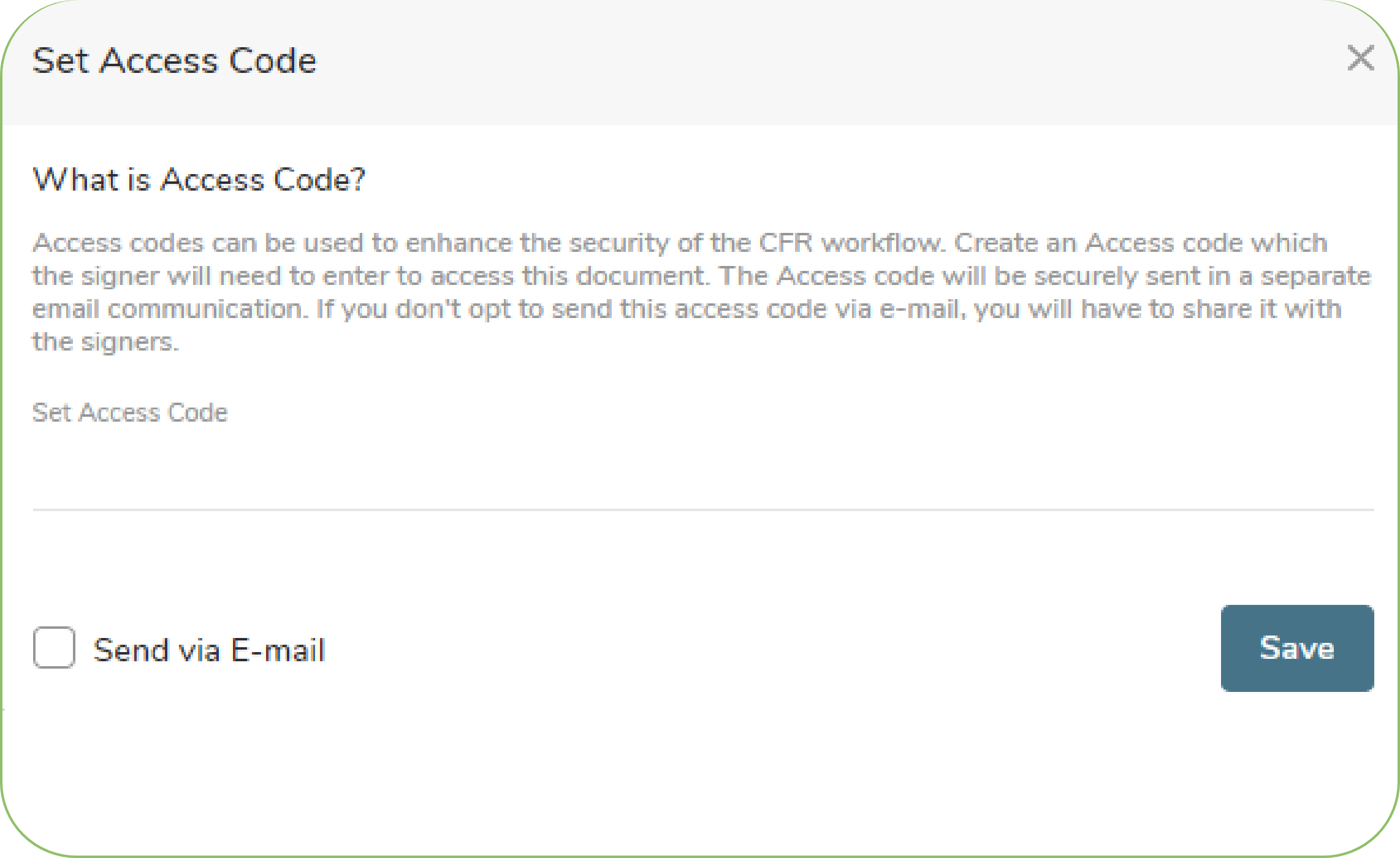
Process flow for signing 21 CFR - PART 11 documents